Abstract
Introduction: Acute myeloid leukemia (AML) is a heterogeneous hematologic malignancy with prognosis and management that is dependent on underlying clinical characteristics, comorbidities, genetic and molecular features. Trisomy 8 is the most frequent numerical chromosome aberration in AML; however, its prognostic significance is not clear. Previous studies reported intermediate prognosis of isolated trisomy 8 (Schoch et al. 1997; Wolman et al. 2002); others concluded that it predicts adverse prognosis (Byrd et al. 1998). We investigated the clinical and genetic characteristics as well as prognostic significance of AML with trisomy 8.
Methods: Clinical and laboratory information of adults with newly diagnosed AML excluding Acute Promyelocytic Leukemia at Beaumont Hospital, an academic medical center from January 2010 to December 2015 were retrospectively reviewed. Statistical analysis was performed using the SAS System version 9.3. Overall Survival (OS) was assessed by Kaplan-Meier method and associations of variables with survival investigated using log rank test and Turkey-Kramer method.
Results:One hundred and thirty-seven AML patients were reviewed. Trisomy 8 was present in the karyotype of 24(18%), 70(51%) had normal karyotype and 43(31%) had other cytogenetic abnormalities without trisomy 8. Median age of patients with trisomy 8 was 71(47-89) and 67(21-91) for others without trisomy 8.
Among the 24 patients with trisomy 8 karyotype, 9(38%) had isolated trisomy 8, 2(8%) had additional single chromosomal abnormality along with trisomy 8, 13(54%) occurred as part of complex karyotype and none was associated with favorable risk cytogenetics, t (8:21) and inversion 16. Secondary AML was present in 12(50%) patients and was preceded by Myelodysplastic Syndrome (MDS) in 11(92%). Only 3(25%) of these secondary AML were observed in the isolated trisomy 8 group. At presentation, 11(46%) of the trisomy 8 karyotype patients presented with leucopenia, 4(17%) had normal white blood cell count (WBC) and 8(33%) with leukocytosis. Hyperleukocytosis, leucocyte count above 100bil/L was present in only 1(4%) patient. The median percentage circulating blast was 10%( 0-95) while median percentage bone marrow blast was 40%( 20-95). Of the 13(54%) patients with trisomy 8 that had molecular testing; Nucleophosmin (NPM1) was negative in 12(92%) while FMS-like tyrosine kinase 3 (FLT3) was negative in 11(84.6%) patients.
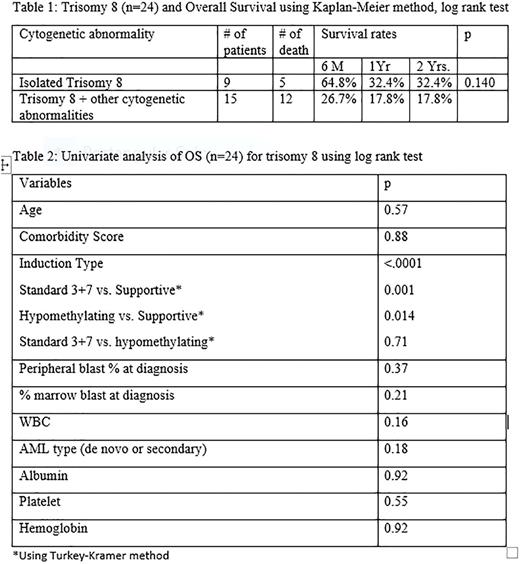
Induction therapy was with 3 days of Anthracycline and 7 days of Cytarabine (3+7) in 12(50%) patients, 5(21%) received hypomethylating agent while 7(29%) had best supportive care. Complete Remission (CR) was achieved by only 5(42%) patients in the "3+7" group, no CR was observed in the other treatment groups. OS appeared better in isolated trisomy 8 relative to combination with other cytogenetic abnormalities though not statistically significant (p=0.140; table 1). Superior OS in patients with trisomy 8 was associated with choice of induction treatment (p<0.0001; table 2). There was no significant association between OS and age, comorbidity score, WBC, percentage circulating or bone marrow blast, platelet count, hemoglobin level, or type of AML (de novo or secondary); table 2.
Conclusion: Adult AML with trisomy 8 is commoner in older age group, with the youngest of our patients being 47 years. It is often preceded by MDS and rarely associated with hyperleukocytosis or high circulating blast. NPM1 and FLT3 mutations were negative in the majority of our patients with trisomy 8, which raises a question about possible mutual exclusion. A trend towards better overall survival was observed with isolated trisomy 8 compared to combination with other cytogenetic aberration though not statistically significant. Our findings are consistent with some prior studies; however larger patient cohort is needed to validate our results.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal